A recently published and peer-reviewed study set out to quantify the amount (if any) of residual DNA in Covid-19 vaccines. There is a specific limit, and there really shouldn’t be any, but if there is, that’s safe, as per the manufacturers, and “experts” such as NZ’s Helen Petousis-Harris.
What the new paper found
A peer-reviewed study just published in Autoimmunity (Speicher, Rose & McKernan, 2025) examined 32 vials of Pfizer and Moderna COVID-19 vaccines from Ontario, Canada. Using two methods — qPCR (which detects specific DNA sequences) and fluorometry (which estimates total DNA) — they reported:
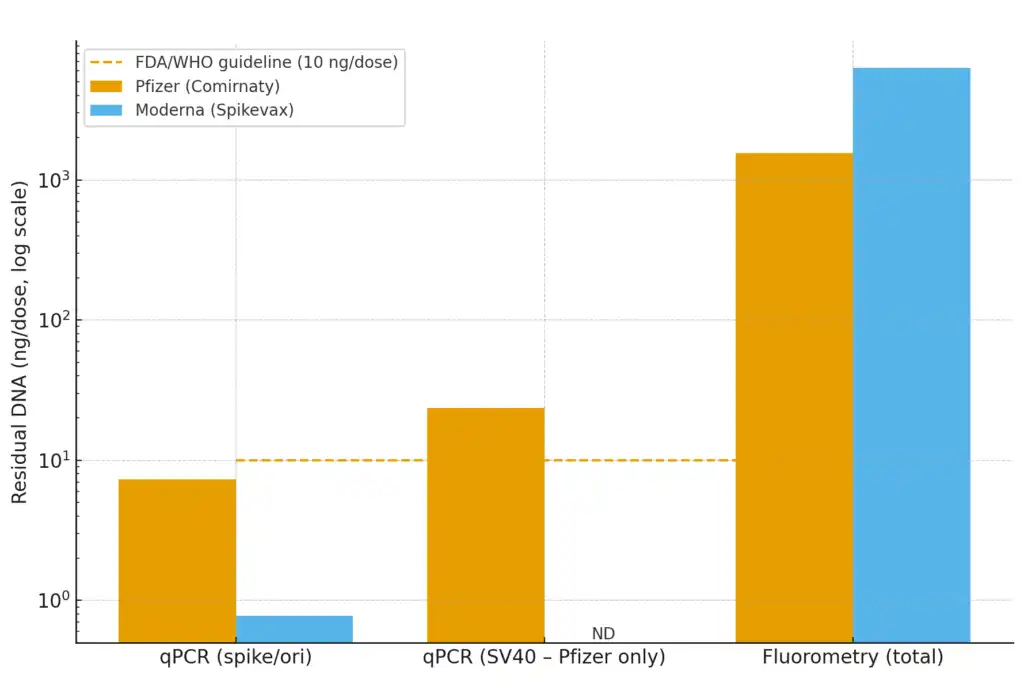
Residual DNA was present in every vial tested.
Pfizer:
qPCR: 0.22–7.28 ng DNA per dose for spike/plasmid regions.
SV40 promoter-enhancer sequence (unique to Pfizer): up to 23.7 ng per dose.
Fluorometry: 371–1,548 ng/dose.
Moderna:
qPCR: 0.01–0.78 ng/dose.
No SV40 sequences detected.
Fluorometry: 1,130–6,280 ng/dose.
DNA fragments averaged ~214 base pairs long, with some as long as 3.5 kb. (or a 3,500 character .txt file)
DNA appeared encapsulated inside lipid nanoparticles, meaning it could enter cells more efficiently.
By qPCR, most vials were within the 10 ng/dose limit set by FDA/WHO. But by fluorometry, all exceeded the limit by 36–627 times.
The authors argued current guidelines were written long before lipid nanoparticles, repeated boosting, or functional sequences like the SV40 enhancer were considered, and may therefore underestimate risk.
What regulators worry about when setting the 10 ng limit
That “≤10 ng” threshold is not a hard poison line. It’s like safe limits for chemicals in drinking water: conservative, with a margin of safety. Risks regulators were guarding against include:
Insertional mutagenesis: DNA integrating into the genome and disrupting normal genes.
Oncogene activation: Strong promoter sequences (like SV40) switching on growth/cancer-related genes.
Chronic inflammation: DNA inside cells triggering innate immune pathways (cGAS–STING).
Clotting risk: Double-stranded DNA fragments affecting blood vessel linings.
Cumulative exposure: Multiple boosters, with DNA shielded in nanoparticles, were not part of the original risk model.
The “other side of the story”
Helen Petousis-Harris, a New Zealand vaccinologist, published an article in October 2024 called Plasmid-gate: Debunking the DNA contamination claims in mRNA vaccines. Her key counterpoints:
DNA fragments are highly degraded and not functional genes.
Amounts detected by reliable qPCR are within regulatory limits.
DNA fragments are destroyed quickly in the body, and integration into the human genome is implausible.
Fears of “turbo cancer” or genomic disruption are unsupported by evidence.
Her view is that such findings are expected “impurities,” not “contamination,” and pose negligible risk within regulatory thresholds.
Importantly, Helen Petousis-Harris isn’t alone in her reassurance. The Australian Therapeutic Goods Administration independently tested batches and confirmed that residual DNA levels are within safety limits. The FDA, Reuters, and vaccine expert Dr. Paul Offit also affirm that there’s no plausible mechanism—or evidence—suggesting residual DNA fragments pose a risk to human DNA or health.
Other Authorities Saying Residual DNA in mRNA Vaccines Is Not a Safety Concern
1. Therapeutic Goods Administration (Australia)
The Australian TGA has reviewed studies claiming excessive DNA in mRNA vaccines and found them methodologically flawed. It conducted its own testing—27 batches tested by qPCR—and confirmed all were within the stringent ≤ 10 ng per dose safety limit. The TGA emphasized that fluorometry can overestimate DNA due to interference from the abundant mRNA, and reaffirmed that there is no evidence of safety risks related to residual DNA .Therapeutic Goods Administration (TGA)
2. International Regulators (e.g., FDA)
In response to concerns about SV40 promoter sequences possibly causing genetic harm, the FDA stated:
Residual DNA in these vaccines is highly unlikely to integrate into human genomes.
Genotoxicity studies in animals show no harmful effects.
Pharmacovigilance in hundreds of millions of recipients indicates no evidence of genomic damage. Therapeutic Goods Administration (TGA)
3. Reuters Fact-Check
A comprehensive Fact-Check by Reuters assessed claims about genomic integration risks and confirmed that experts and regulators agree that there is no evidence supporting risk from residual DNA fragments in mRNA vaccines. Reuters
4. Scientific Experts (e.g., Dr. Paul Offit & Scientific American)
Renowned vaccinologist Dr. Paul Offit has explained that the chances of vaccine DNA altering human DNA are virtually nonexistent—you’d have a better chance of becoming Spider-Man than suffering harm from such DNA fragments. Scientific American
Summary Table: “Other Side” Authorities on Residual DNA Safety
| Authority / Expert | Summary Position |
|---|---|
| Australian TGA | Found all tested mRNA vaccine batches met DNA safety guidelines via qPCR; fluorometry method issues are known. |
| US FDA | Asserted residual DNA is unlikely to integrate, found no genotoxicity in animal studies, and no safety signals in humans. |
| Reuters Fact-Check | Consensus among experts and regulators: residual DNA poses no integration or health risk. |
| Dr. Paul Offit (Scientific American) | Residual DNA poses no danger to human DNA integrity; safety concerns are implausible. |
Where this leaves us
The bottom line is not that one side is “right” and the other “wrong,” but that:
Residual DNA fragments are present in both Pfizer and Moderna vaccines.
Quantification depends heavily on the method used (qPCR vs fluorometry).
Regulatory thresholds were set for an older generation of vaccines and may need updating for lipid nanoparticle delivery platforms.
Debate remains active: some scientists call for stricter oversight and transparency, others insist current limits are adequate.
For SpiderCatNZ, the guiding principle remains the same as when plotting those first per-capita charts: show the data as it is, without assumptions. Readers can then decide what the numbers mean for themselves.
Sources
Speicher, D.J., Rose, J., & McKernan, K. (2025). Quantification of residual plasmid DNA and SV40 promoter-enhancer sequences in Pfizer/BioNTech and Moderna modRNA COVID-19 vaccines from Ontario, Canada. Autoimmunity, 58(1), 2551517. DOI: 10.1080/08916934.2025.2551517
Petousis-Harris, H. (2024). Plasmid-gate: Debunking the DNA contamination claims in mRNA vaccines. Global Vaccine Data Network.